What is a GMP audit and what organizations do they apply to?
The United States FDA developed good manufacturing practice regulations in response to laws enacted by the US Congress. These laws were developed in response to increasing public concern and scrutiny of food and drug manufacturers, prompted by numerous scandals and instances of malfeasance. Some of these events included poisonings, deaths, lifelong deformities, and outbreaks of infection. Today, numerous standards bodies and governments publish GMP guidelines and regulations. Depending on the source, the GMPs may be legally enforceable or simply good practices where the manufacturer gains a marketing edge by demonstrating that they follow the standards. For example, a medical device manufacturer in the US must follow the FDA GMP regulations governing safety and effectiveness. A cosmetics manufacturer wishing to import into a European Union country must follow the GMP guidelines of the European Union. Both of these sets of GMPs have the force of law since they are regulations. As another example, a cosmetics manufacturer operating in India might follow the international standard “ISO 22716 Cosmetics — Good Manufacturing Practices (GMP) — Guidelines on Good Manufacturing Practices.” This is a GMP standard, not a regulation, which means that following it helps ensure quality, but it does not equate to a legal standard. To further complicate things, a GMP standard may be more exacting than the law, or vice versa. In addition, a country’s law may exceed the standard, or the standard may exceed the law, and both may cite each other in their texts. Lastly, a government or standards body may publish an additional layer of “harmonizing” documents intended to reconcile all these regulations and standards and make them easier for various manufacturers to understand and follow.Why conduct a Good Manufacturing Process Audit?
A GMP audit is an essential part of the overall quality program at a supplier. A GMP audit is a systematic examination of an organization’s practices as they relate to the relevant standard or regulation. Ideally, this audit is conducted by a third party to attain objectivity and detachment. The audit should also go beyond simply listing nonconformances but also include potential opportunities for improvement (OFI). The objective of the audit is typically to indicate the overall state of compliance, allowing a partner to decide if and how to continue the business relationship. The audit findings must ultimately result in actions to fix nonconformances and prevent a recurrence.What to expect during the GMP audit
A well-conducted GMP audit will include:Opening meeting
The opening meeting is the place to discuss the audit plan and make arrangements for tours and other inspections. An auditee point person or representative will make introductions and provide any other needed logistical info. It is a good time to reiterate the scope of the audit (what standards and regulations will be audited against) and any areas of emphasis (such as follow-up items from a previous audit or client-specific production lines). This is also the time to envoke a friendly, collaborative tone and rapport, set aside a conference room as the day’s home base, and get any other company or personnel information needed for the audit report.Tour of production facilities
A tour may be done first in the day, last, or not at all in the case of a paper audit. During the pandemic era, some audits went entirely remote, with company personnel carrying a live camera while the auditor watches and asks questions from their office. The tour may focus on a client-specific production area or a testing area. It may focus on packaging, shipping, labeling or any number of other areas. The auditor will, with advance permission, photograph relevant areas to support the audit report, such as critical machinery, testing areas, signs of pests, the exterior of the building, or the production line.Examination of records
A record is generally defined as a historical file that indicates what was done. Examples include a maintenance log on a critical machine, a calibration log, or a cleaning log. Under GMP, these are often subject to retention schedules. If an auditor asks for 5 years of calibration logs for a piece of testing equipment, these records will provide objective evidence of the equipment’s state of calibration during that period. Another example is employee training checklists, certificates and diplomas. Records point to who did what and when.Documents
A document is generally defined as a content file that is editable and revisable under a controlled system of revision. A standard operating procedure, a corporate policy manual, or a facility map are all documents. An auditor will examine these documents to assess compliance with GMP. For example, if the relevant GMP standard requires that a quality control unit be established and documented, then the auditor will ask for the documents (such as SOPs and testing protocols) that this unit uses in their work.Interviews with relevant employees
The auditor may interview production line staff or management. Auditors will ask clarifying questions about procedures and training. Some auditees may choose to make only management available for interviews. Others may freely allow frontline staff to be interviewed. It is important to note that an auditor is not looking for “gotcha” moments or finding fault, but rather to gain an objective view of the state of GMP compliance of the organization. Thus, an auditee should give employees basic coaching on how to answer an auditor’s questions, but not train them to be tight-lipped or to give canned answers.Closing meeting
The closing meeting is where the day wraps up and any preliminary audit findings and observations are presented. Serious findings should be brought up immediately and formally detailed later in the audit report that will follow. This is the time to thank all participants and end on a good note, with an estimate of when to expect the report.Example areas audited for GMP compliance
An audit against the US FDA’s GMP requirements for medical devices will include the following areas:- Document controls
- Records control
- Device master record
- Device history record
- Contract review
- Design controls
- Purchasing controls
- Control of customer-supplied product
- Identification and traceability
- Production and process controls
- Process validation
- Receiving, in-process and finished product testing
- Device labeling
- Nonconforming product
- Corrective and preventive action
- Device packaging, handling and shipping
- Quality audits
- Statistical techniques
Example of a useful audit finding, recommendation, and follow up
The purpose of a GMP audit is to provide an assessment of the company’s state of GMP compliance and ultimately lead to corrective and preventive actions addressing any nonconformances. The following is a finding from a Pro QC audit report on a cosmetics manufacturer. Pro QC Report Example – Audit Score and Summary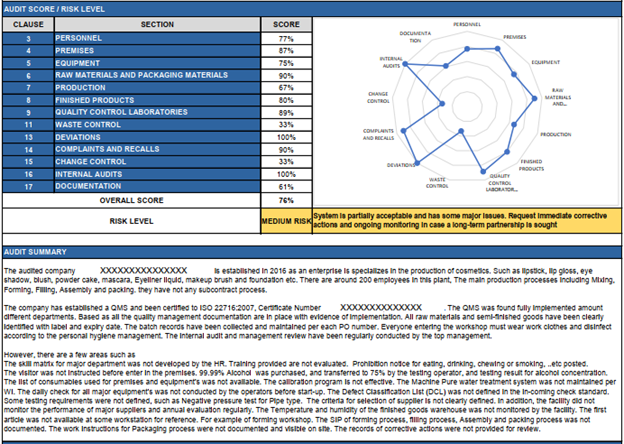 Pro QC Report Example – Recommended Corrective Actions
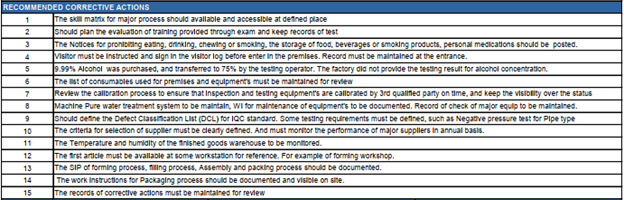
Pro QC Report Example – Recommended Corrective Actions
 Applicable standard
ISO 22716:2007 Cosmetics — Good Manufacturing Practices (GMP) — Guidelines on Good Manufacturing Practices, Section 4.7
Questionnaire item
Is there adequate lighting, that is sufficient for operations, installed in all areas?
Evidence
1) Record of light intensity.
2) Device to measure light
Findings
The lighting intensity looks sufficient at the moment. However, there is no device for measuring the intensity, no plan and no record.
Score
I (improvement needed)
In this example, the audit report cited the applicable standard the factory was audited against, cited objective evidence (a light measuring device), the finding, and an indication of the state of compliance (compliant, needs improvement, or non-compliant). The audit report highlights this potential nonconformance in yellow and ranks it according to severity to allow the auditee to act in a prioritized manner.
Applicable standard
ISO 22716:2007 Cosmetics — Good Manufacturing Practices (GMP) — Guidelines on Good Manufacturing Practices, Section 4.7
Questionnaire item
Is there adequate lighting, that is sufficient for operations, installed in all areas?
Evidence
1) Record of light intensity.
2) Device to measure light
Findings
The lighting intensity looks sufficient at the moment. However, there is no device for measuring the intensity, no plan and no record.
Score
I (improvement needed)
In this example, the audit report cited the applicable standard the factory was audited against, cited objective evidence (a light measuring device), the finding, and an indication of the state of compliance (compliant, needs improvement, or non-compliant). The audit report highlights this potential nonconformance in yellow and ranks it according to severity to allow the auditee to act in a prioritized manner.
What to expect in a Pro QC GMP audit report
The audit sponsor can expect a comprehensive and organized report with a statement of overall GMP compliance of the auditee, detailed and prioritized audit findings, and recommended corrective actions. The report will include a recommendation on the risk involved in partnering with the audited supplier. See Pro QC’s suite of GMP audit services and sample audit reports here.Contact Us
Click Below to Improve Product Quality with Pro QC
Contact usGet a Quote or Book a Service
We will be happy to give you more information about our quality solutions. Please fill in this form to contact us. We will respond as soon as possible.
If you would like to submit your CV, please click here.
Contact Us by Email
Contact Us by Phone
North America
+1 206 397 1145
Mexico
+52 55 9990 7885
Colombia
+57 601 9190355
United Kingdom
+44 330 094 5589
France
+33 9 7303 6784
Germany
+49 15630 810151
Australia
+61 2 8252 7691
India & South Asia
+91 227 189 7407
Asia & Asia Pacific
+886 2 2832 2990
Stability
Pro QC has been providing quality assurance, engineering and consulting services since 1984.
Global Presence
We offer a dedicated Account Manager and quality solutions in over 100 countries.
Timely Feedback
We offer online reporting and detailed reports within 24 hours of job completion.
Quality Experts
Our global engineering team has the requisite industry expertise to verify quality and conformance.