COVID-19 – Medical Device & PPE Supplier Verification & Inspection Service
Pro QC protects buyers from defective PPE and compliance risks by providing supplier verification and rigorous product inspections
Due to the COVID-19 virus, the world is desperately scrambling for Medical Personal Protective Equipment (PPE). Whether for personal, commercial or medical use, everyone is competing for a limited supply of legitimate PPE products and services.
This is a desperate time for both buyers and sellers. We have already witnessed numerous cases of fake suppliers and scams resulting in huge losses on both sides, and in some cases, those losses have reached one million USD. As a comprehensive answer to this challenge, we have developed a COVID-19 Supplier Verification and Inspection service that has already helped numerous clients prevent some of the following supply chain challenges:
- Opportunistic supplier or reseller scams
- PPE purchases from unauthorized suppliers
- Defective, contaminated and dysfunctional medical PPE
- Custom clearance issues due to government restriction or invalid product certification
- Unexpected quality costs due to quality and production issues
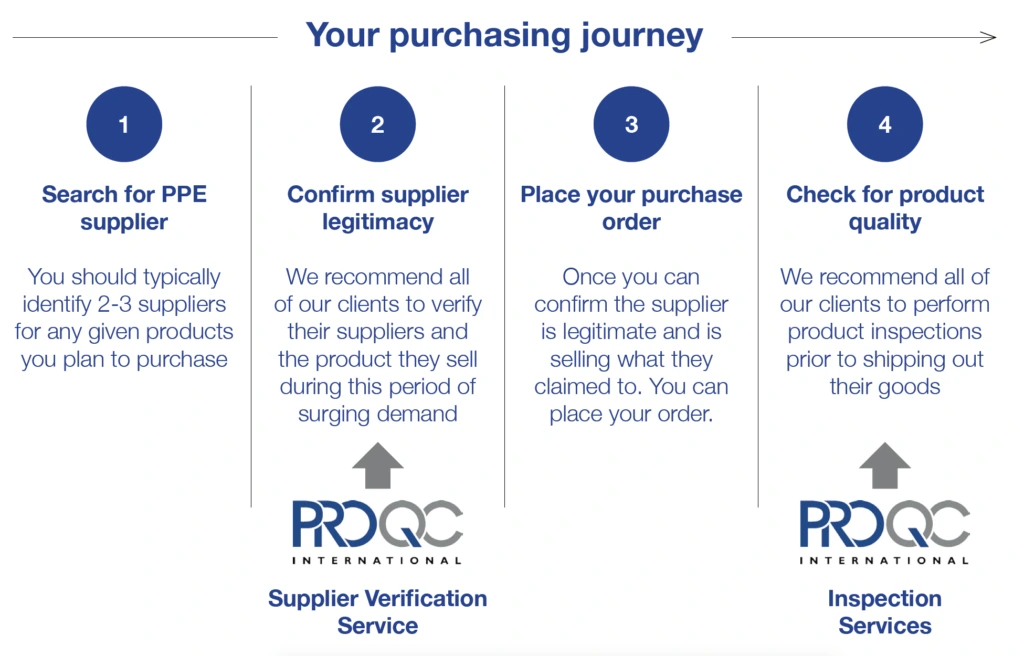
The graphic below shows where Pro QC can support buyers along the purchasing journey and avoid the costly challenges listed above.
Contact Us
COVID-19 – Medical Device & PPE Supplier Verification Service
Pro QC urges all buyers to remain vigilant especially when purchasing medical PPE from a new or untested supplier. Our COVID-19 Supplier Verification service will ensure that a stringent process is followed to verify any new supplier and then ensure rigorous quality controls remain in place until the goods are loaded for shipment. All our on-site evaluations and reports are prepared by our ISO 13485 certified, GMP auditors.
What is included in our Supplier Verification Report?
- Pro QC’s risk evaluation & recommendation
- Supplier profile
- Factory business license
- Factory standard certifications (i.e. ISO 9001:2015, ISO 13485:2016, GMP for Medical Devices, others…)
- Product certification research, checking and verification against FDA, CE and other market regulations
- General production environment observations
- PPE product catalogue, certificate, quantity in-stock, quantity per package
- Legal export locations & existing product certificates
- Current factory staffing capacity
- Other general issues
- Photo evidence and report
Off-site (Remote) Supplier Verification for urgent purchase order
In general, on-site supplier verification is recommended and requires approximately three to five days to arrange. However, we understand urgent cases arise and as a response we provide an off-site Supplier Verification Service. Although not as detailed as the on-site service, it can be more time and cost effective for our client. Pro QC has a strict photo evidence guideline for accepting remote photo evidence from suppliers which ensures the authenticity of the photo. If the case is urgent, ask about our off-site or remote Supplier Verification service.
COVID-19 – Medical Device & PPE Inspection Service
Once a supplier’s authenticity and capacity has been assured, Pro QC recommends a product inspection before the goods leave the supplier’s facility. The third-party inspection will the verify product conformance to design and quality requirements. Pro QC has experience inspecting a wide range of personal protective equipment & medical devices including:
- PPE Masks (Single use & surgical medical mask, and pollution masks)
- PPE Gloves (Medical gloves – latex, vinyl & other protective gloves for professional use)
- Protective Clothing & Footwear (medical gowns, life vests & high-visibility vests)
- Eye & Face Shields
- COVID – 19 Test Kits
- Other PPE for Leisure Purposes (bike helmets, diving suits and many others)
Pro QC follows the International Inspection Standard ANSI Z.1.4-2003 for sampling. Inspection plans include general quality criteria covering appearance, functionality, packaging integrity, workmanship, and other custom requirements. PPE products are also inspected against product and region-specific standards such as EN14683, ASTM F2100 & NIOSH for medical masks; ASTM D5151, EN 455 for medical gloves and EN 13795 & ASTM F2407 for medical gowns. Noteworthy quality inspection checkpoints are included in the table below.
| Medical Masks | Medical Gloves | Medical Gowns |
|---|---|---|
|
|
|
A multifaceted approach to quality assurance of Personal Protective Equipment begins with verification of the supplier, then the inspection of the products, and is completed with container loading supervision. For more information or pricing contact us using the forms at the side or bottom of the page.


Pro QC International
Our Service Reports
Detailed & Actionable
- Following International & Industry Standards
24 Hours
- Report Delivery forInspections
48-72 Hours
- Report Delivery for Audits
Why Pro QC International?
Advanced Quality Analysis
Industry-leading quality dashboard, analytics, and supplier performance analysis tools and framework
Expert-Led Solutions
Technical expert advice from first contact ensures reliable solutions are executed with precision
Qualified Resources
Resource transparency and the right qualifications safeguard your interests on every project at every supplier
Globally Aligned Operations
Operational processes are optimized and aligned for a seamless cross-regional experience
Stringent Technical Oversight
Meticulous technical preparation, guidance throughout the engagement, and report review by senior engineers
Single Contact Point
A single contact point for quality services globally simplifies your supply chain
Stability and Expertise
Founded in 1984, over 40 years of experience make Pro QC a trustworthy and dependable quality partner
Pro QC Blog Posts & Related Offerings
Find out more about our related services by clicking below:
Click Below to Improve Product Quality with Pro QC
Contact usGet a Quote or Book a Service
We will be happy to give you more information about our quality solutions. Please fill in this form to contact us. We will respond as soon as possible.
If you would like to submit your CV, please click here.
Contact Us by Email
Contact Us by Phone
North America
+1 206 397 1145
Mexico
+52 55 9990 7885
Colombia
+57 601 9190355
United Kingdom
+44 330 094 5589
France
+33 9 7303 6784
Germany
+49 15630 810151
Australia
+61 2 8252 7691
India & South Asia
+91 227 189 7407
Asia & Asia Pacific
+886 2 2832 2990
Stability
Pro QC has been providing quality assurance, engineering and consulting services since 1984.
Global Presence
We offer a dedicated Account Manager and quality solutions in over 100 countries.
Timely Feedback
We offer online reporting and detailed reports within 24 hours of job completion.
Quality Experts
Our global engineering team has the requisite industry expertise to verify quality and conformance.